Optimizing IVF Success: Understanding the Critical Role of Retrieval Timing and Protocol
- Staff Writer
- Mar 4
- 5 min read
The precise timing of the ovulation trigger remains one of the most critical decisions in an IVF cycle, directly impacting both the number and quality of retrieved oocytes. Administering the trigger too early—before follicles reach a suitable size (typically <15mm)—can result in immature oocytes (not yet at the MII stage), requiring in vitro maturation with less predictable outcomes. Conversely, delaying the trigger until follicles exceed 22mm risks retrieving post-mature, “overcooked” oocytes that may have compromised fertilization potential.
Individual variations in hormonal dynamics and follicular development necessitate tailored monitoring and decision-making, especially in the final days of stimulation. It is during this period that the interplay of medications and endogenous hormonal fluctuations reaches its peak complexity, demanding not only scientific rigor but clinical experience and, at times, instinct.
A Real-Life Example: Illustrating the Complexity
Consider a healthy 41-year-old patient with diminished ovarian reserve (AMH ~0.6). On day 9 of ovarian stimulation (day 14 of her natural cycle), her lead follicle measured 16mm. Although this size typically justifies triggering, several trailing follicles measured only 10–11mm. Meanwhile, estradiol levels had more than doubled over two days (from 195 to >400 pg/mL), signaling active follicular development. However, LH had risen to 15 mIU/mL, indicating a risk of premature ovulation.
Previously, a follicle measuring around 15mm at the time of trigger on day 12 of medication had resulted in an euploid embryo. This suggests that smaller follicles might yield higher quality eggs for this patient and adds complexity to the decision. The clinician faced a challenging choice: trigger immediately to ensure retrieval of the largest follicle, potentially sacrificing the smaller follicles, or delay triggering to allow additional follicular growth, at the risk of an LH surge causing premature ovulation and the leading follicles past its prime time.
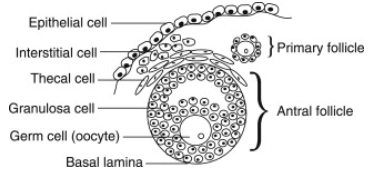
Why Follicle Size Matters at the Time of Trigger
Typically, fertility doctors in most clinics in the world aim to trigger ovulation when the lead follicles are at 18-22mm size (Table 1). This textbook guidance does not take into consideration the pace follicles grow, nor the age of the patients. Emerging evidence suggests a more individualized approach may yield superior outcomes.

A study of 11,462 patients in 2023 suggest that for young patients (< 35 years), triggering when there is a high proportion of large or medium follicles results in better quality oocytes; while for older patients (≥ 35 years), it is better to trigger when the proportion of medium follicles is no less than that of small follicles. At the same time, a study by Weill Cornell Medicine concluded that the ideal time to trigger is when the median of the three largest follicles is ≥20 mm, particularly in patients of advanced maternal age. These divergent recommendations highlight how much discretion and clinical judgment is required in practice.
Intricate dance of LH and FSH prior to retrieval
Luteinizing hormone (LH) naturally initiates ovulation, but an unplanned LH surge can prematurely release oocytes, resulting in an unsuccessful retrieval. To suppress this, clinicians typically use GnRH antagonists such as Ganirelix or Cetrotide, which inhibit LH and FSH secretion via direct pituitary suppression.
Ganirelix or Cetrotide bind competitively and directly to pituitary GnRH receptors, immediately
suppresses both LH and FSH to prevent early ovulation. Unfortunately, this suppression of FSH—critical for follicular support—necessitates compensatory FSH supplementation through additional Gonal-F and/or Menopur injections to boost the FSH at the same time. These two sets of injections have conflicting impact on FSH, but are often administered together out of doctors’ despair to trick our bodies and keep all the hormones at their desired level.
Emerging Alternatives: The Role of Orilissa
Orilissa (Elagolix), an oral GnRH antagonist, presents an alternative less intrusive and cheaper option. Orilissa also blocks pituitary GnRH receptors directly, but its oral bioavailability allows systemic distribution and dose-dependent suppression. Unlike injectables, Orilissa enables partial suppression of LH while preserving some FSH activity, supporting ongoing follicular development without risking premature ovulation. As a result, Orilissa effectively controls LH levels without dramatically suppressing FSH, potentially simplifying hormone management.
Triggering Ovulation: More Decisions to Make
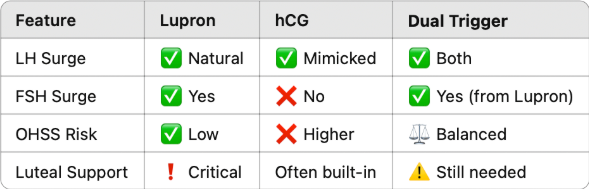
Once follicles reach optimal sizes, ovulation will be triggered. There are two types of triggers and both are injections - Lupron and hCG triggers. Doctors often prescribe to one of them, or sometimes both at the same time.
Lupron stimulates the pituitary to release a natural LH surge and a smaller FSH surge that mimics the body’s natural mid-cycle, triggering a series of events in the ovary: the final maturation of oocytes, cumulus expansion (the process where cumulus cells, surrounding the oocyte, change from a compact mass to a dispersed structure, producing a hyaluronan-rich extracellular matrix (ECM), crucial for oocyte maturation and fertilization), and oocyte detachment from follicle wall. The whole process takes about 35-36 hours after the trigger injection.
The other option is hCG triggers, which directly binds LH receptors on granulosa and theca cells, which surround the oocytes in a follicle, without stimulating the pituitary gland. After hCG injection, oocyte matures, cumulus expansion happens, and granulosa cells start progesterone production. hCG has a longer half-life than Lupron, which allows it to continue stimulating corpus luteum to support pregnancy. At the same time, there is a risk of overstimulating the ovaries post-retrieval.
Depending on the patients’ profiles and how they respond to hormone stimulations, doctors choose either approach or both sometimes. When used together, it is called a dual trigger, which most doctors believe can create the best of both worlds to increase mature oocyte yield, improve fertilization rates, and boost embryo quality. But there is no one size fits all formula. Which trigger to use should be decided on a case by case basis. hCG helps to prepare for implantation, which might not be necessary for patients who do not intend implantation or who want to do IVF treatment continuously.
Sometimes, ibuprofen, such as Motrin Aleve or Advil, are used to delay ovulation if an early LH surge is detected, allowing for a more controlled timing of the egg retrieval procedure.
Timing Egg Retrieval: Striking a Critical Balance
Conventionally, egg retrieval is scheduled approximately 35-36 hours after the trigger shot(s). This specific timing balances the need to maximize follicle maturation against the risk of premature ovulation. Studies have shown that follicles continue to grow 1-2mm after trigger and undergo final maturation. Waiting too long increases the risk of premature ovulation, while scheduling retrieval too soon might yield immature oocytes. The fear of not catching the eggs before they are released makes almost all doctors stick to the 35-36 hour window.
However, if monitored even closer, doctors could conduct staggering retrievals, sometimes 3-4 hours apart, so that the mature oocytes can be captured while giving time for smaller follicles to further develop. While this strategy may improve yield, it is time- and labor-intensive, and thus uncommon in clinical settings.
Final Thoughts: Why Personalization Is Paramount
Despite IVF’s standardized appearance, the final days of stimulation are anything but routine. They are marked by volatile hormonal shifts, daily decisions, and a need for deep clinician-patient collaboration. Protocol flexibility and responsiveness in this phase can significantly influence outcomes.
No single trigger timing or hormone strategy fits all. Embracing personalization—grounded in real-time data, clinical expertise, and patient context—is essential to optimize IVF success and retrieve the best possible oocytes.




Comments